Small molecule binding to surface-supported single-site transition-metal reaction centres
Result of the Month
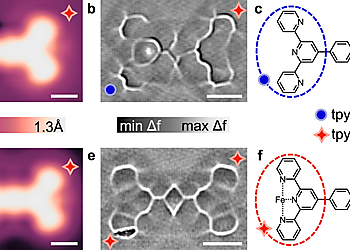
In order to study how a noble metal surface would modify a ligand-bound metal site’s reactivity, terpyridine-phenyl-terpyridine (TPT) molecules containing one (a–c) and two (d–f) active Fe-tpy sites are prepared on Ag(111) surface. Bare terminal terpyridine (tpy) and Fe-tpy have different appearances in STM and ncAFM images.
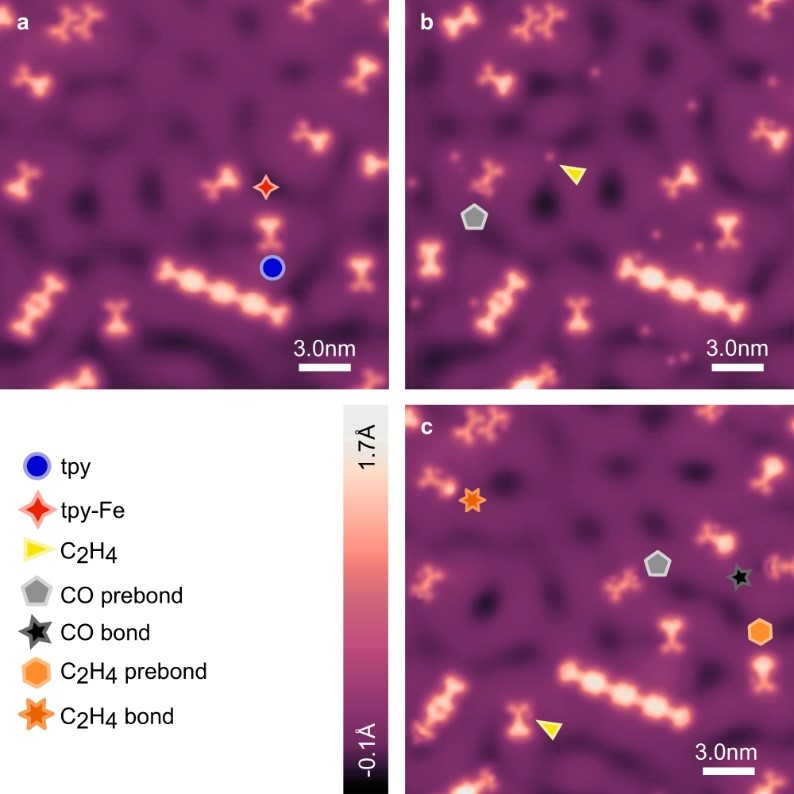
fig 2. STM overviews of reaction progression. STM topographs of the same region of a sample imaged after active Fe-tpy site preparation (a), after subsequent exposure to CO and C2H4 (maximum temperature during dosing 11.1 K) (b), and after successive incremental sample annealing up to T = 30 K for t = 5 min (c). Free C2H4 (yellow triangle) is visible on the surface, along with changes to active sites labelled “CO prebond” (grey pentagon), after gas exposure (b). After subsequent annealing of the sample at T = 30 K for t = 5 min (c) three additional distinct changes to active tpy-Fe sites are visible and labelled: “CO bond” (black five point star), “C2H4 prebond” (light orange hexagon), and “C2H4 bond” (orange six point star). After the anneal, C2H4 molecules can form pseudo hydrogen bonds with the nitrogen lone pairs of the distal, outward pointing pyridyl of non-metalated tpy groups (c, yellow triangle). Images shown were acquired with a metal tip at V = 20 mV and I = 20pA (a–c).
To explore reactivity, STM and ncAFM observations were carried out at three stages: (a) after active Fe-tpy site preparation on Ag(111). Bare tpy and tpy-Fe sites can be distinguished. (b) after the prepared Fe-tpy sites were exposed to low concentrations of gaseous CO and C2H4 via a leak valve. Isolated C2H4 molecules and CO molecules pre-bonded to Fe-tpy sites are shown. (c) after subsequent annealing, changes to the physical and electronic structures of the active sites are revealed, namely the CO prebond, CO bond, C2H4 prebond and C2H4 bond configurations.
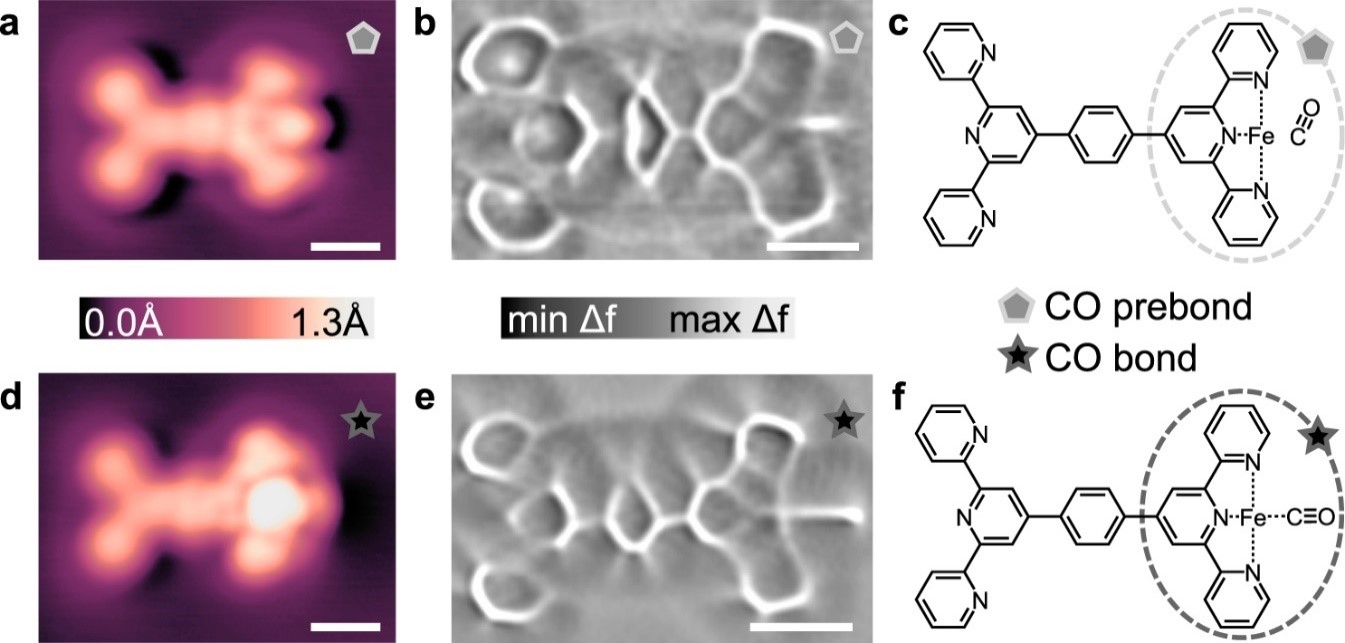
fig 3. Comparison of CO prebond (a–c) and CO bond (d–f) configurations. STM topographs (a, d) were imaged with CO-terminated tips at V = 20 mV and I = 10 pA. Constant height ncAFM images (Laplace-filtered) for the CO prebond (b) and CO bond (e) motifs show a distinct difference in the length of lines at the right side of the molecules where the CO is located. The proposed chemical structures for the CO prebond and CO bond configurations are shown in (c) and (f), respectively. Parameters for (b): V = −0.95 mV, Avib = 2 Å, ∆z = −0.01 nm from setpoint V = 20 mV and I = 10 pA on Ag(111). Parameters for (e): V = −0.95 mV, Avib = 8 Å, ∆z = −0.06 nm from setpoint V = 20 mV and I = 10 pA on Ag(111). Scalebars: 6.0 Å (a, d) 5.0 Å (b, e).
High resolution STM and ncAFM were used to resolve the difference between CO prebond and CO bond configurations. The different appearances are attributed to the CO molecule being in a “stand-up” geometry in the prebond configuration and being in a “lay-down” geometry in the bonded configuration. DFT simulations confirmed this point.
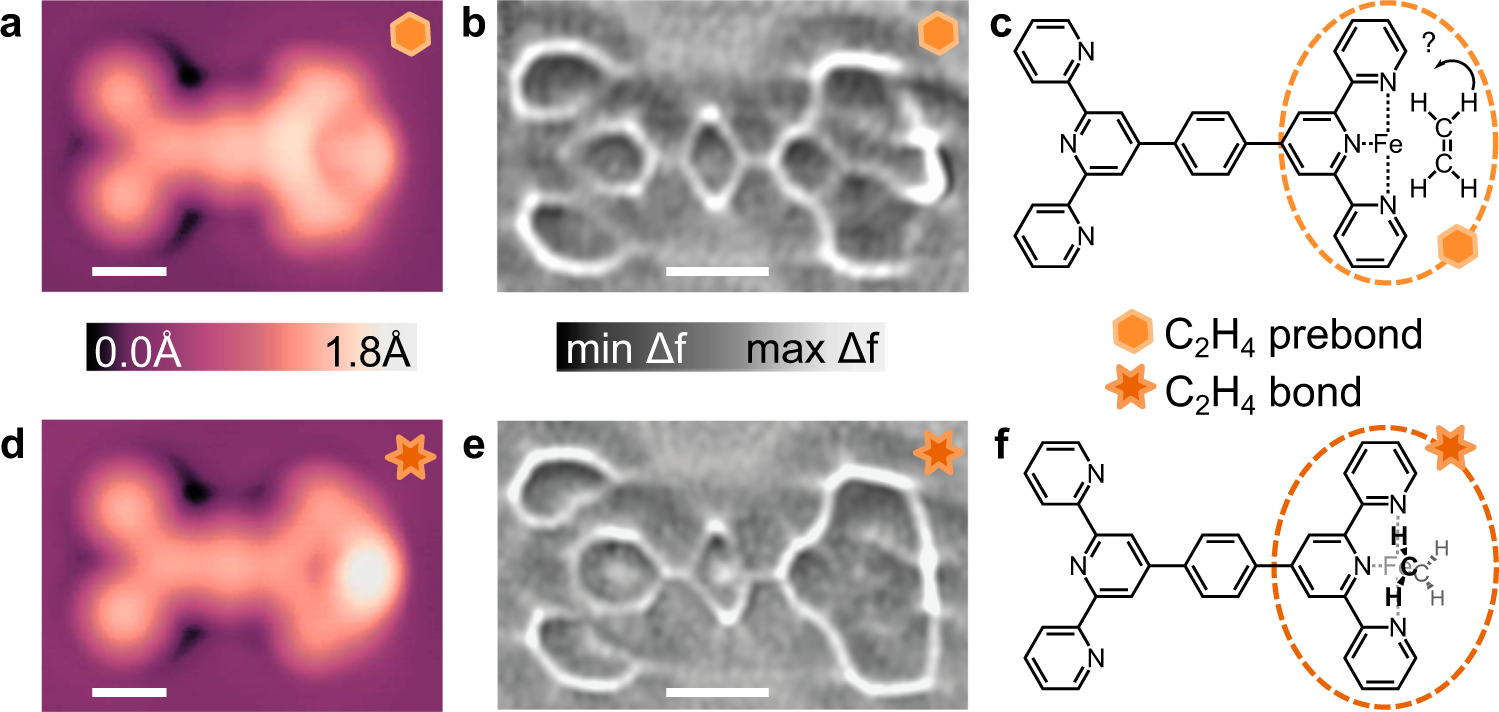
fig 4. Comparison of C2H4 prebond (a–c) and C2H4 bond (d–f) configurations. Topographs of the C2H4 prebond (a) and C2H4 bond (d) configurations imaged at V = 20 mV and I = 10pA. Laplace-filtered ncAFM images of the C2H4 prebond (b) and C2H4 bond (e) motifs imaged at constant height with Avib = 2 Å from a setpoint of V = 20 mV and I = 10 pA on Ag(111). ∆z = −0.01 nm for (b), and ∆z = −0.022 nm for (e). Images (a, b, d, e) were all acquired with the same CO-terminated tip. Shear transformation performed on (b) to compensate for drift. Proposed chemical structures for the horizontal C2H4 prebond and vertical C2H4 bond motifs drawn in (c) and (f), respectively; note for the pre-bond structure, surface adsorption would indicate a flat-lying structure as shown, while DFT shows a weakly bonded side-orientation. Scalebars 5 Å (a, b, d, e).
High resolution STM and ncAFM images similarly show differences between C2H4 prebond and C2H4 bond configurations. In contrast to the situation of CO molecule, C2H4 adsorbs with its π-system parallel to the Ag surface, providing a hypothesis for the pre-bond structure. However since some heat is required (through in-situ annealing) to allow C2H4 to diffuse, it has some energy to re-arrange and partially rotates at the Fe site as determined by simulations in good agreement with the experimental data. Once the C2H4 molecule’s π-system is bonded to the Fe atom, the C2H4 molecule “stands up” and becomes orthogonal to the tpy ligand plane similar to the expected gas-phase configuration. This is also confirmed by DFT stimulation.
All STM and ncAFM data were acquired at 4.2 K in a Scienta Omicron LT-SPM. NcAFM measurements were performed with CO-terminated W tips on a homebuilt qPlus sensor. To terminate the tip with CO, df(z) sweeps incrementing 1 Å from an initial ∆z = 5 Å were performed over a single CO molecule until it was transferred to the tip.