Direct observation of glycans bonded to proteins and lipids at the single-molecule level
Result of the Month
Scienta Omicron Fermi SPM is used to image glycoproteins for the first time, enabling their structural analysis for the first time. Glycoproteins are proteins decorated with glycans (a.k.a. carbohydrate) that influences many biological functions and dysfunctions in living systems. Structural analysis of glycoproteins at single molecule level has been long sought due to the loss of structural information when these molecules are subjected to ensemble-averaged analysis. Direct imaging by STM allows the structures and locations of glycans on proteins to be determined at the single molecule level, breaking the analytical barriers in the field of glycoscience.

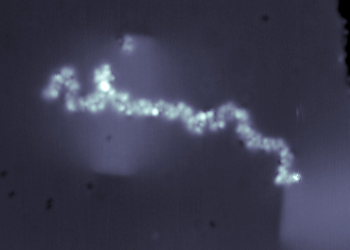
Constant current STM image (8 x 8 nm2) of a glycan-decorated lipid (i.e. GD3 ganglioside) on Cu(100) surface imaged at 11 K sample temperature, 300 fA current setpoint, and +0.3 V surface bias.
GD3 Ganglioside is one of the glycolipids (i.e. glycan-decorated lipids) that have been found abundantly on the surfaces of cancer cells, including breast cancers, bladder cancers, and lung cancers.
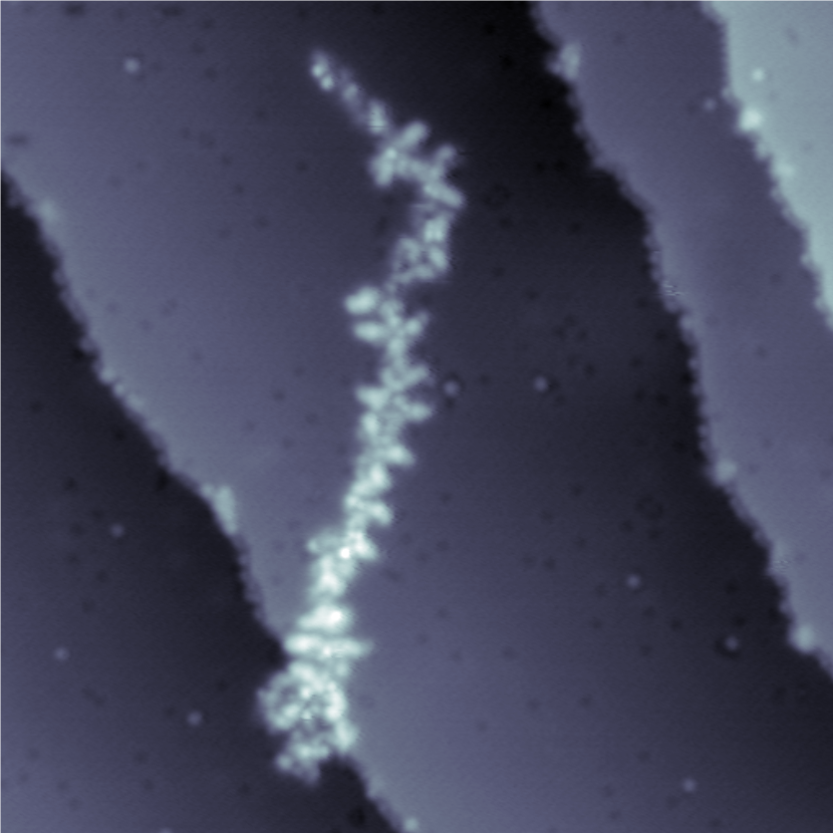
Constant current STM image (47 x 47 nm2) of a glycan-decorated protein (i.e. MUC1) on Cu (100) surface imaged at 11 K sample temperature, 300 fA current setpoint, and +0.3 V surface bias.
MUC1 is a glycoprotein found abundantly on the surfaces of breast cancer cells, which has been marked as the US NIH National Cancer Institute as one of the priority cancer antigens.